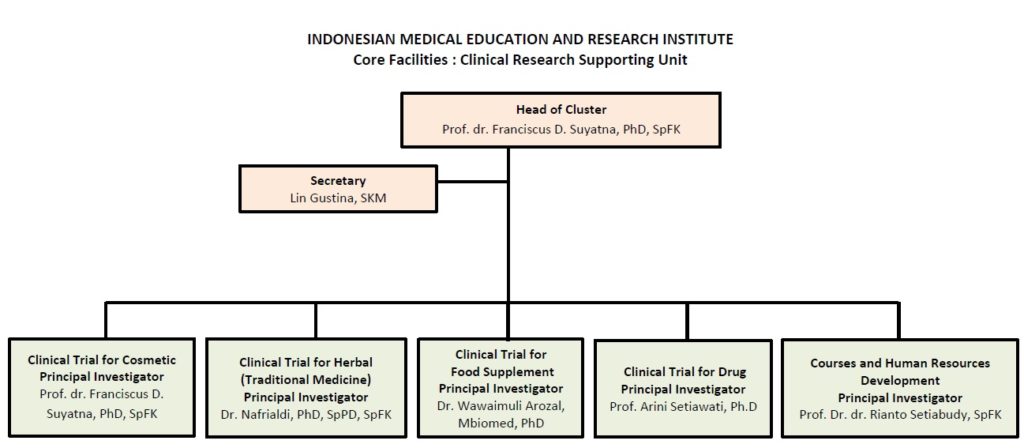
Clinical Research Supporting Unit (CRSU)
Is a contract research organization (CRO) with the main mission to organize and conduct clinical studies in Indonesia and overseas and ensure that they are in accordance with International Conference Harmonization and Good Clinical Practices (ICH-GCP). CRSU has a good networking with industry and related institutions both in Indonesia and overseas. CRSU mainly focuses on improving the quality of clinical trials in Indonesia through conducting clinical research according to GCP guidelines. In line with this, CRSU has established good collaboration with various stakeholders.
Ongoing Research
- Biosimilar clinical trial of erythropoietin.
- Patch Test Study: Sensitization and Hypoallergenic for Baby Products.
- Clinical trial of infertility supplement.
Publication
Collaboration
- Pharmacies
PT. Novartis.
PT. Bayer.
PT. Sanofi Aventis.
PT. Otsuka.
PT. Abbot.
PT. Takeda.
PT. Daewoong.
PT. Roche.
PT. Morinaga.
Kohjin Life Sciences Co., Ltd.
Alert Asia.
PT. BSN.
OrchidLife Sdn Bhd.
PT.Astra Zeneca.
PT. Dexa Medica.
PT. Rohto.
PT. Etana.
PT. Sari Husada Generasi Mahardhika.
PT. Ika Pharmindo.
PT. Kino Care.
PT. Deltomed Lab.
PT. Kalbe Pharma.PT.
Tempo Research.PT.
Mecosin Indonesia.PT.
Novell Pharmaceutical Lab.
PT. Sanghiang Perkasa.
PT. Combiphar.
PT. Soho Industri Pharmacies.
PT. Fukuhara Health Care Indonesia.
PT. Triyasa Nagamas Farma. - Hospital
RSUPN DR. Cipto Mangunkusumo.
RSUP Dr. Kariadi.
Pusat Jantung Nasional Harapan Kita.
RS Kanker Dharmais.
RSIA Budi Kemuliaan.
Klinik Makara Universitas Indonesia. - University
Sekolah Farmasi Institut Teknologi Bandung.
UIN Syarif Hidayatullah Jakarta. - Laboratory
Laboratorium Prodia.
Pharma metric.
Equilab.
Laboratorium Terpadu FKUI.
Organization